Characterization of the Physicochemical and Thermal properties of the Biofield Energy Treated Flutamide Using PSA, PXRD, TGA/DTG, and DSC Analytical Techniques- Juniper Publishers
Juniper Publishers- Journal of complementary medicine
Abstract
Flutamide is an antiandrogen drug that blocks the
action of testosterone by binding to the androgen receptor. This study
was designed to determine the impact of the Trivedi Effect®-Energy of
Consciousness Healing Treatment on the physicochemical and thermal
properties of flutamide. The test sample was divided into two parts,
i.e., control and treated sample. The control part was known as
untreated sample, while the treated part remotely received the Biofield
Energy Healing Treatment by a renowned Biofield Energy Healer, Alice
Branton. The study showed that the particle size values were
significantly increased by 15.82%(d10), 16.36%(d50), 1.05%(d90), and
5.10% {D (4, 3)}; thus, the specific surface area was significantly
decreased by 14.56% in the treated sample compared with the control
sample. The PXRD peak intensities and crystallite sizes were
significantly altered ranging from 7.02% to 29.41% and -9.17% to 17.86%,
along with 2.84% increase in the average crystallite size in the
treated flutamide compared to the control sample. The residue weight was
significantly decreased by 64.16%; however, the maximum thermal
degradation temperature was increased by 10.16% in the treated sample
compared to the control sample. The latent heat of the treated sample
reduced by 9.37% compared with the control sample. The results revealed
the significant alteration in the crystallinity, particle size and
thermal stability of the treated sample as compared to the untreated
sample. Thus, the Biofield Energy Treated flutamide might improve the
flowability, and compatibility compared with the untreated sample, that
may help in designing a better pharmaceutical formulation in terms of
its performance against various diseases.
Keywords: Flutamide,
The trivedi effect®, Energy of consciousness haling treatment,
Complementary and alternative medicine, PSA, PXRD, TGA, DSC
Introduction
Flutamide is a nonsteroidal pure antiandrogen drug
(toluidine derivative, structurally associated to bicalutamide and
nilutamide), which perform its action by inhibiting the uptake and/or
binding of dihydrotestosterone to the target cell receptor that leads to
alter the interfering with the androgen action. It blocks the action of
both endogenous and exogenous testosterone by binding to the androgen
receptor, thus flutamide administration results in elevations of plasma
testosterone and estradiol [1]. However, it was reported that it is a
potent inhibitor of testosterone-stimulated prostatic DNA synthesis.
2-Hydroxyflutamide, an active metabolite of flutamide competitively
blocks the dihydrotestosterone binding at androgen receptors, which
results in the formation of inactive complexes that could not be
translocate into the cell nucleus. This property is significantly useful
to arrest the growth of tumour cell or transient tumour regression [2].
Absorption, distribution, and excretion of flutamide is very rapid and
absorbed
completely. In addition, it is rapidly and extensively metabolized with
only 2.5% of plasma radioactivity of 1 hour after administration. The
biological half-life of the alpha-hydroxylated metabolite of flutamide
is approximately 6 hours. Flutamide, as an anti-androgens action used to
treat prostate cancer in men, by blocking the effects of testosterone
that helps prostate cancer to grow and also significantly used with
other medications for radiation treatments [3]. Flutamide is
administered and preferred orally, while it is 95% plasma protein-bound
and seems to concentrate in the prostate. The data suggested that
flutamide undergoes rapid metabolism to a variety of compounds. However,
95% of an oral dose is excreted by the kidneys. Hemodialysis cannot
remove it due to its high protein binding. It might have some side
effects because this is commonly used with other medications, such as
hot flashes, diarrhoea and nausea, loss of sexual interest/ability,
vomiting, and enlargement of male breasts. However, drowsiness is one of
the less common side effects, while diarrhoea is a common side
effect. The rate of absorption and mechanism of action depends
upon various factors of drugs such as its solubility, stability,
pharmacokinetics
and bioavailability [4,5]. However, physicochemical
properties of any pharmaceuticals are very important in the different
role for its biological profile. Therefore, in order to improve
the physiochemical profile such as such as particle size, crystalline
structure, crystallite size, surface area, etc., research has been
carried
to alter the physicochemical properties.
The Biofield Energy Treatment is considered as an emerging
field as it is an integral healthcare approach including the
increasing beneficial effects of Complementary and Alternative
Medicine (CAM) therapies, against various health conditions
[6,7]. National Institute of Health (NIH) recommend and included
various Energy Healing therapies such as natural products, yoga,
deep breathing, meditation, homeopathy, progressive relaxation,
acupressure, acupuncture, hypnotherapy, relaxation techniques,
healing touch, pilates, Ayurvedic medicine, traditional Chinese
herbs and medicines, Reiki, cranial sacral therapy, etc. under CAM
category and these are accepted by most of the U.S. population
with several advantages [8,9]. Similarly, the Biofield Energy
Healing (the Trivedi Effect®) has also been popular worldwide
due to its remarkable impact on the nonliving materials as well
as the living organisms. The Trivedi Effect®-Consciousness
Energy Healing Treatment has been reported for its significant
impact on the physicochemical and thermal properties of various
pharmaceutical/nutraceutical compounds [10-12], plants [13,14],
altered characteristics in microbiology [15-17], metals, ceramics,
and polymers [18,19], livestock [20], biotechnology [21], and
skin health [22]. Thus, this study was aimed to determine the
effect of the Biofield Energy Treatment (Trivedi Effect®) on the
physicochemical and thermal properties of flutamide by using
various analytical techniques such as, particle size analysis (PSA),
powder X-ray diffraction (PXRD), thermogravimetric analysis
(TGA), and differential scanning calorimetry (DSC).
Materials and Methods
Chemicals and reagents
Flutamide was purchased from Tokyo Chemical Industry Co.
Ltd. All other chemicals used during the experiments were of analytical
grade available in India.
Consciousness energy healing treatment strategies
Flutamide, i.e., the test compound was divided into two parts.
Among both parts, one portion was denoted as control sample
that did not receive the Biofield Energy Treatment. Besides, the
other part of flutamide was considered as the treated part that
received the Energy of Consciousness Healing Treatment by the
renowned Biofield Energy Healer, Alice Branton (USA), and named
as the Biofield Energy Treated sample. In the process of Biofield
Energy Treatment, the sample was kept under the standard laboratory
conditions, and the Biofield Energy Healer provided the
Trivedi Effect® - Energy of Consciousness Healing Treatment to
the sample, remotely, for 3 minutes through the Unique Energy
Transmission process. On the other hand, the control flutamide
was subjected to a “sham” healer under the similar laboratory
conditions, who did not have any knowledge about the Biofield
Energy Healing Treatment. Consequently, the control as well as
Biofield Energy Treated flutamide samples were kept in similar
sealed conditions and further characterized by using modern analytical
techniques.
Characterization
The PSA, PXRD, TGA/DTG, and DSC analysis of pyridoxine
were performed. The PSA was performed using Malvern Mastersizer
2000, from the UK with a detection range between 0.01μm
to 3000μm using the wet method [23,24]. The PXRD analysis of
pyridoxine powder sample was performed with the help of Rigaku
MiniFlex-II Desktop X-ray diffractometer (Japan) [25,26]. The average
size of crystallites was calculated from PXRD data using the
Scherrer’s formula (1)
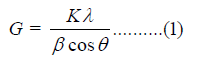
Where G is the crystallite size in nm, k is the equipment constant
(0.94), λ is the radiation wavelength (0.154056nm for K α
1 emission), β is the full-width at half maximum, and θ is the
Bragg angle [27]. Similarly, The TGA/DTG thermograms of pyridoxine
were obtained with the help of TGA Q50 TA instruments.
The DSC analysis of pyridoxine was performed with the help of
DSC Q200, TA instruments [23,24]. The % change in particle size,
specific surface area (SSA), peak intensity, crystallite size, melting
point, latent heat, weight loss and the maximum thermal degradation
temperature (Tmax) of the Biofield Energy Treated sample was
calculated compared with the control sample using the following
equation 2:
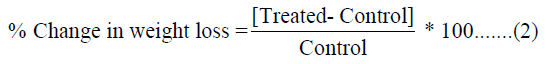
Results and Discussion
Particle size analysis (PSA)
The particle size analysis of the control and Biofield Energy
Treated samples were presented in Table 1. The particle size distribution
of the control sample was found at d10 = 44.31μm, d50
= 179.98μm, d90 = 653.57μm, and D (4, 3) = 276.60μm. However,
the particle size distribution of the Biofield Energy Treated flutamide
sample was observed at d10 = 51.32μm, d50 = 209.43μm,
d90 = 660.45μm, and D (4, 3) = 290.70μm. The result analysis revealed
that the particle size values at d10, d50, d90, and D (4, 3) in
the Biofield Energy Treated sample were significantly increased
by 15.82%, 16.36%, 1.05%, and 5.10%, respectively, compared to
the control sample.
On the other hand, the specific surface area of the Biofield Energy
Treated flutamide (0.088m2/Kg) was decreased by 14.56%
as compared with the control sample (0.103m2/Kg). The literature
reported the impact of particle size distribution of drug on the formulation development in terms of its blend uniformity,
compactibility, and flowability, etc., which further affected the
safety, efficacy, and the quality of the formulation [28,29]. Hence,
the Biofield Energy Treated flutamide sample might help in better
formulation development by improving its uniformity, flowability,
and compactibility.
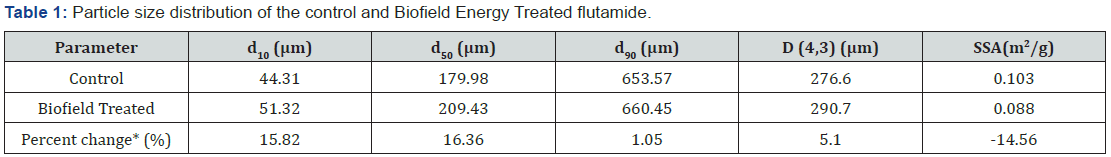
d10, d50 and d90:
particle diameter corresponding to 10%, 50%, and 90% of the cumulative
distribution, D (4,3): the average mass-volume diameter,
and SSA: the specific surface area. *denotes the percentage change in
the Particle size distribution of the Biofield Energy Treated sample
with
respect to the control sample.
Powder X-ray Diffraction (PXRD) Analysis
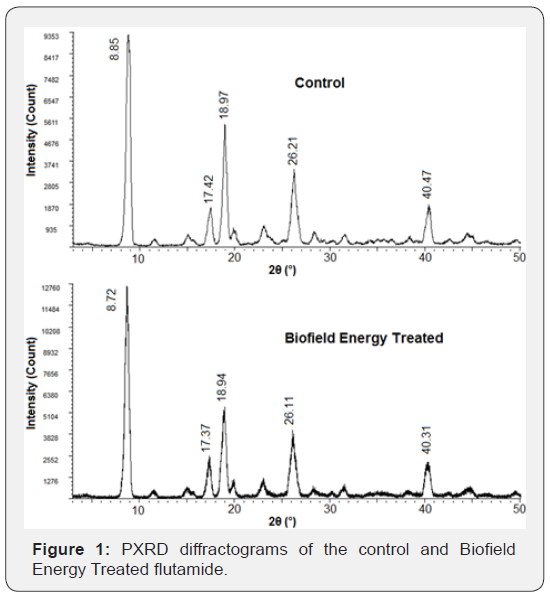
The PXRD diffractograms of the control and Biofield Energy
Treated flutamide samples are shown in Figure 1. There was the
presence of sharp and intense peaks in the diffractograms of both
the samples which indicated that both the samples are crystalline
in nature. Besides, the peak intensities and the crystallite size corresponding
to each characteristic peak was done for both the control
and the Biofield Energy Treated sample (Table 2).
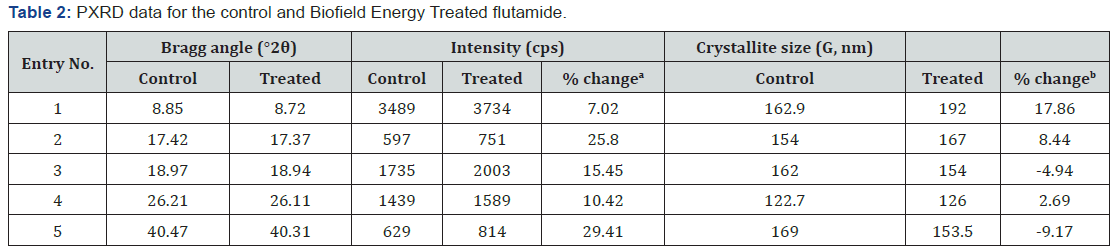
a: Denotes the percentage change in the peak intensity of Biofield Energy Treated sample with respect to the control sample.
b: Denotes the percentage change in the crystallite size of Biofield Energy Treated sample with respect to the control sample.
The highest peak intensity (100%) was observed at 2θ equal
to 8.85° (Table 2, entry 1) in the PXRD diffractogram of the control
sample, while at 8.72° in the Biofield Energy Treated sample; however,
the Bragg’s angle of all the characteristic peaks of the Biofield
Energy Treated sample were observed to differ from the control
sample. Also, the peak intensities corresponding to these characteristic
diffraction peaks in the Biofield Energy Treated sample
were found to be significantly increased ranging from 7.02% to
29.41% compared to the control sample. Such alterations in the
peak intensities of the peaks indicated the change in the crystallinity
of the Biofield Energy Treated sample as compared to the
control flutamide sample.
Besides, the crystallite sizes of the Biofield Energy Treated
sample corresponding to those peaks were also observed to be
significantly altered ranging from -9.17% to 17.86% as compared
to the control sample. Also, the Biofield Energy Treated sample
showed an increase in the average crystallite size (158.5nm) by
2.84% as compared to the control sample (154.12nm). The literature
reported that the alterations in the peak intensity of the crystalline
compound changes based on its crystal morphology [30].
Moreover, the alterations in the complete PXRD pattern may be
considered as the proof of polymorphic transitions taken place in
treated flutamide sample [31,32]. Thus, the overall results indicated
the alterations in the crystallinity, crystallite size, and polymorphic
form of the Biofield Energy Treated flutamide sample when
compared with the control sample. Such changes might ensure its
better drug performance in the formulation development than the
untreated flutamide.
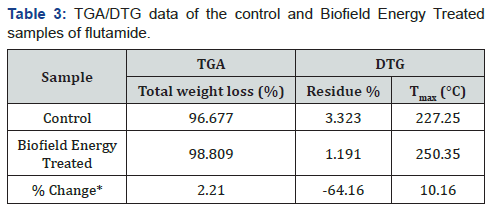
Thermal gravimetric analysis (TGA)/ Differential thermogravimetric analysis (DTG)
The TGA thermograms of the control and Biofield Energy
Treated flutamide samples displayed one step of thermal degradation
(Figure 2). The results revealed 2.21% increase in the total
weight loss of the Biofield Energy Treated flutamide as compared
with the control sample (Table 3). Also, the residue amount of the
treated flutamide sample was reduced significantly by 64.16%
when compared to the control sample (Table 3).
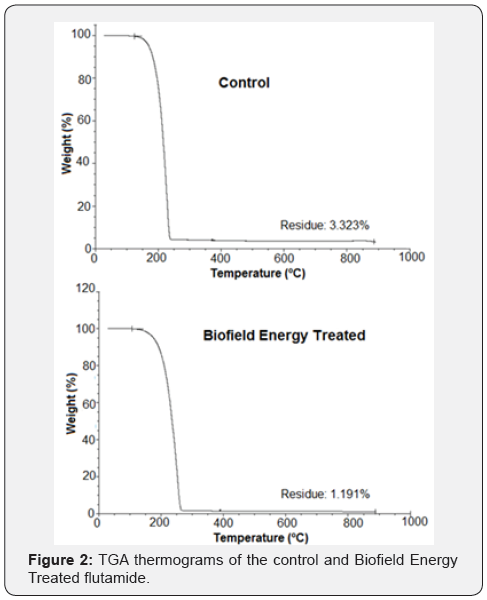
*denotes the percentage change of the Biofield Energy Treated sample
with respect to the control sample,
Tmax = the temperature at which maximum weight loss takes place in TG
or peak temperature in DTG.
The DTG thermograms of the control and Biofield Energy
Treated sample showed a single peak (Figure 3). The results
revealed that the onset of thermal degradation taken place at
134.68°C in the control flutamide sample, while it started earlier
in the Biofield Energy Treated sample i.e., at 103.41°C. However,
the maximum thermal degradation temperature (Tmax) of the
treated flutamide sample was significantly increased by 10.16%
as compared with the control sample (Table 3). Thus, the overall,
TGA/DTG results showed that the thermal stability of the Biofield
Energy Treated sample was significantly decreased as compared
with the control flutamide sample.
Differential scanning calorimetry (DSC) analysis
The DSC thermograms of both the control and the Biofield Energy
Treated flutamide samples were shown in Figure 4 and the
results were used to determine the melting and other thermal behaviours
of the flutamide sample [33]. A sharp endothermic peak
was evident in the thermograms of both the samples which are
considered as the melting temperature of the samples. The peak
was observed in the control sample at 113.23°C; while it was
slightly decreased to 112.58°C in the Biofield Energy Treated flutamide
sample. Moreover, the latent heat of fusion (ΔH) of the con trol sample was found as 107.2 J/g; whereas it was decreased to
97.15J/g in the Biofield Energy Treated sample. Hence, the results
revealed a reduction in the melting point and the ΔHfusion of the Biofield
Energy Treated flutamide by 0.57% and 9.37%, respectively
as compared to the control sample (Table 4). It is presumed that
there was might be some alterations in the molecular chains and
the crystallization structure of the flutamide [33] due to the Biofield
Energy Treatment that may cause the changes in the melting
temperature and ΔH of the treated flutamide.


ΔH: Latent heat of fusion, *denotes the percentage change of the
Biofield Energy Treated sample with respect to the control sample.
Conclusion
The study revealed that the Trivedi Effect®-Consciousness
Energy Healing Treatment showed a significant impact on the
particle size distribution, crystallite sizes, peak intensities, and
the thermal properties of flutamide. The particle size values of
the Alice’s Biofield Energy Treated flutamide were increased
significantly by 15.82%, 16.36%, 1.05%, and 5.10% at d10, d50,
d90, and D (4, 3), respectively compared to the control sample.
The specific surface area of the Biofield Energy Treated sample
was found to be decreased by 14.56% compared to the untreated
flutamide sample. The increase in particle size might be helpful
in providing the better compactibility, uniformity, and flowability
to the treated flutamide sample. The PXRD results showed
alterations in the Bragg’s angle of the highest intensity peak
as well as the other peaks of the treated sample. Also, the peak
intensities and crystallite sizes corresponding to those peaks of
the treated flutamide sample showed alterations ranging of 7.02%
to 29.41% and -9.17% to 17.86%, respectively as compared
to the untreated sample. Similarly, the average crystallite size
of the Biofield Energy Treated flutamide sample was also
increased by 2.84% compared to the control sample. Besides,
the total weight loss of the Biofield Energy Treated sample was
increased by 2.21% in TGA; whereas, the residue amount was
significantly reduced by 64.16% as compared with the control
sample. The DTG study showed that the Tmax of the Biofield Energy
Treated flutamide sample was significantly improved by 10.16%
compared with the control sample. The Biofield Energy Treated
sample revealed that the melting temperature and ΔHfusion were
decreased by 0.57% and 9.37%, respectively as compared to the
untreated flutamide sample. Thus, the thermal analysis indicated
the less thermal stability of the Biofield Energy Treated sample
as compared to the control sample. Overall, the Trivedi Effect®-
Consciousness Energy Healing Treatment poses its impact on
the flutamide sample, which might generate its new polymorphic
form with altered crystallinity, particle size, and thermal stability.
Such alterations in the Trivedi Effect® Treated flutamide may
confirm its better designing in the form of nutraceutical and
pharmaceutical formulations by providing better compactibility,
flowability, and content uniformity, which might be used to offer
better therapeutic response against prostate cancer, androgendependent
skin and hair conditions including acne, seborrhea,
hirsutism, and scalp hair loss, hyperandrogenism, as well useful
for feminizing hormone therapy aimed at transgender women.




Comments
Post a Comment