Antioxidant, Antimicrobial And Wound Healing Activity Of Salvadora persica Twig Extracts- Juniper Publishers
Juniper Publishers- Journal of Complementary Medicine
Abstract
Wound healing is a complex multifactorial process
that results in the contraction and closure of the wound and restoration
of a functional barrier. Salvadora persica, commonly known as Miswak
was found to contain constituents such as tannins, saponins, flavonoids
and sterols. Hence it is thought to evaluate wound healing activity of
Salvadora persica since phytoconstituents like tannins, saponins and
flavonoids are known to promote the wound healing process due to their
antioxidant and antimicrobial activities. Antimicrobial and antioxidant
activities of Salvadora persica extracts were studied to understand
mechanism of wound healing process. Total phenolic content (TPC) was
estimated to screen the prepared extracts by using Folin-Ciocalteu
phenol reagent method. Methanol extract showing highest TPC was
undertaken for detailed antioxidant, antimicrobial and wound healing
activities. Methanol extract showed moderate antioxidant activity on
scavenging DPPH, ABTS radicals and by pyrogallol red bleaching method.
Methanol extract also showed antimicrobial activity against wound
pathogens by agar diffusion method. Methanol extract was formulated into
gel and wound healing activity of gel was evaluated using incision and
excision wound models in rats. Topical application of prepared gel on
the excision wound in rats caused higher rate of contraction and reduced
the period of epithelialization when compared to control group animals.
In incision wound model, breaking strength of animals treated with the
gel containing methanol extract of Miswak twig was found to be
significantly (p < 0.001) higher as compared to the control group
animals. Hence, the present study revealed that gel containing methanol
extract of Miswak twig possess wound healing activity.
Keywords:Incision wound;
Excision wound; Antioxidant; Antimicrobial activity; Phytomedicines;
Cholesterol plasma levels; DPPH; Ascorbic acid Abbrevations: TPC: Total Phenolic Content; DPPH: 1,1-Diphenyl-2-picryl-hydrazy; AA: Ascorbic Acid; IAEC: Institutional Animal Ethic Committee
Introduction
Wound healing is an interaction of complex cascade of
cellular and biochemical actions healing to the restoration of
structural and functional integrity with regain of strength of injured
tissues [1]. It involves continuous cell - cell interaction and cell
matrix interactions that allow the process to proceed in different
overlapping phases including inflammation, wound contraction , re
epithelialization, tissue remodeling and formation of granulation tissue
with angiogenesis [2]. These events are regulated by several mediators
including platelets, inflammatory cells, cytokines and growth factors,
and matrix metalloproteinases and their inhibitors [3].
Several factors such as bacterial infection,
oxidative stress, necrotic tissue and interference with blood supply,
lymphatic blockage and disease condition such as diabetes mellitus delay
or reduce the wound healing process. Generally, if the above factors
could be altered by any agent, an increased healing rate could be
achieved [4].
Nature has gifted us with many herbs having mystical
healing properties that are used widely in number of ailments.
The use of herbs and medicinal plants as the first medicine is a
universal phenomenon. Today, as much as 80% of the world’s population
depends on traditional medicine as primary health care needs [5]. Many
Plants and their extracts being antioxidant and/or antimicrobial actions
have immense potential for the management and treatment of wounds. The
phytomedicines for wound healing are not only cheap, well tolerated and
affordable but are also purportedly effective and safe as hyper
sensitive reactions are rarely encountered with the use of these agents
[6]. These natural agents induce healing and regeneration of the lost
tissue by multiple mechanisms. Herbal medicines in wound management also
involve disinfections, debridement and provide a moist environment to
encourage the establishment of the suitable environment for natural
healing processes [7].
Salvadora persica (family Salvadoraceae) is an
upright evergreen small tree or shrub. It is commonly known as Miswak
or Tooth brush tree and is widely distributed in India, Africa, Saudi
Arabia, Iran, Israel and Pakistan. It has been claimed in traditional
literature to be valuable against a wide variety of diseases [8].
The traditional medicinal use of Salvadora persica as
antimicrobial toothbrush stick for oral hygiene, and to treat gum
inflammation, is a centuries old practice and a part of Greeko-
Arab system of medicine [9]. Pharmacological studies indicated
that Salvadora persica L. plant possess anti-microbial, anti-plaque,
aphrodisiac, alexiteric, analgesic, anti-inflammatory, anti-pyretic,
astringent, diuretic and bitter stomachic activities. It has great
medicinal use in the treatment of nose troubles, piles, scabies,
leucoderma, scurvy, gonorrhea, boils and toothache, to treat
hook worm infections, venereal diseases, for teeth cleaning, in
rheumatism, cough and asthma, to lower cholesterol plasma
levels, reestablishment of the components of gastric mucosa, and
as a laxative [10]. It contains important phytoconstituents such
as vitamin C, salvadorine, salvadourea, alkaloids, trimethylamine,
cyanogenic glycosides, tannins, saponins and salts mostly as
chlorides [11].
However, there is no previous report on wound-healing
activity of Salvadora persica twig in literature. The purpose of the
present study was to investigate in vivo wound healing activity of
Salvadora persica twig. Since antioxidant and antimicrobial agents
play an important role in wound healing process, antioxidant and
antimicrobial activities of Salvadora persica twig were carried out
to find the mechanism behind wound healing process.
Materials and Method
Materials
Gallic acid, 1,1-Diphenyl-2-picryl-hydrazy (DPPH), ascorbic
acid (AA), Folin-Ciocalteu phenol reagent, pyrogallol red, 2,2’azinobis
(3- ethylbenthiazoline-6-sulphonic acid) (ABTS), potassium
persulphate and all other substances used were obtained from
Sigma- Aldrich Co. Ltd. Nutrient agar was obtained from Himedia
(Mumbai, India). All chemicals used, including the solvents were
of analytical grade.
Plant materials
The twig of Salvadora persica were collected from Malvani
area in Malad, Mumbai, India and authenticated by Agharkar
Institute, Pune, India.
Methods
Extraction of plant material
Authenticated plant material was further dried in shade,
powdered and used for extraction. Extraction was carried out using
various solvents such as Petroleum ether (60-800), chloroform,
methanol, 50% aqueous alcohol and water. The extracts were
concentrated in a rotary evaporator under pressure, were kept in
desiccators and used for further studies.
Determination of extractive value
10 gm of powdered material were extracted with 100ml
solvent using Soxhlet extraction apparatus. The % yield of each
extract was determined.
in vitro antioxidant assay methods
Phenolic compounds could be a major determinant of
antioxidant potentials of foods and could therefore be a natural
source of antioxidants [12]. Hence total phenolic content of the
prepared extracts was determined to screen the bioactive extract.
Determination of total phenolic content (TPC)
The total phenolic content was measured using Folin-
Ciocalteau reagent as per procedure described by Singleton et
al., with some modifications [13]. Test mixture consists of 1ml
of extract solution (0.1 or 1mg/ml), 0.5ml of Folin Ciocalteau
reagent and 5ml of distilled water. The mixture was incubated at
room temperature for 10min. Then 1.5ml of anhydrous sodium
carbonate solution (10% w/v) was added and the final volume was
made upto 10ml. The final mixture was allowed to stand at room
temperature for 2 hr. The absorbance was measured at 725nm
using UV-Vis spectrophotometer. The experiment was carried out
in triplicate. Gallic acid was used for preparing the standard curve
(10μg/ml to 100μg/ml). The total phenolic content in the plant
extract was expressed as milligrams of gallic acid equivalent per
gram of dry weight (mg GAE/g) of extract.
The extract showing maximum TPC was further used for
various in vitro antioxidant assays, antimicrobial activity and in
vivo pharmacological activities.
DPPH radical scavenging activity
The free radical scavenging activity of active extract was
measured by DPPH using the method of Blios [14]. An aliquot
of 1ml of the extract solution in various concentration range
was added to 3 ml of 0.1 mM DPPH solution. The decrease in
the absorbance was determined at 517 nm after 30 min. The
percentage scavenging activity was calculated from [(A0-A1)/A0]
× 100, where A0 is the absorbance of the control, and A1 is the
absorbance of the extract/ standard. A blank is the absorbance
of the control reaction (containing all reagents except the test
compound). The % scavenging activity and IC50 value of each
extract was calculated for the various concentrations. Ascorbic
acid was used as standard antioxidant for comparison.
Peroxynitrite pyrogallol red bleach method
Pyrogallol Red solution (100μM) was prepared in 100mM
phosphate buffer, pH 7.4. 1ml of extract solution was added to
2ml of 100μM Pyrogallol Red solution.0.5ml of 200μM/liter
peroxynitrite solution was added to the mixture and vortexed
immediately. After 15 minutes the absorbance was measured
using UV-Vis spectrophotometer at 540nm. The % inhibition
of pyrogallol red bleaching was determined using the formula
[(A1-A2)/A1] X 100, where A1 is the absorbance in presence of
antioxidants and A2 is the absorbance in absence of antioxidants.
The IC50 values yielding 50% inhibition of Pyrogallol Red
bleaching were estimated. Ascorbic acid was used as standard
antioxidant for comparison [15].
ABTS assay
ABTS was dissolved in water to a 7mM concentration. ABTS
radical cation (ABTS+.) was produced by reacting ABTS stock
solution with 2.45mM potassium persulfate and allowing the
mixture to stand in the dark at room temperature for 12-16 hr. The ABTS+. solution was diluted with a phosphate buffer (2mM,
PH 7.4) to achieve an absorbance of 0.8 ± 0.014 at 734nm.
Extract solutions were mixed with ABTS+. solution, and after 1
min the absorbance was read using UV-vis spectrophotometer
at 734 nm. Phosphate buffer solution was used as a blank. The
% radical-scavenging activity of the samples was determined
using the formula [(Acontrol-Atest)/ Acontrol] X 100, where Acontrol is the
absorbance of the control (ABTS+• solution without test sample)
and Atest is the absorbance of the test sample (ABTS+• solution with
extract). The IC50 values scavenging 50% of ABTS+. were estimated.
Ascorbic acid and Trolox were used as standard antioxidants for
comparison [16].
Antimicrobial activity
in vitro antibacterial and antifungal activities of methanol
extract of bark of Miswak twig were determined by the agar
diffusion method against wound pathogens [17]. Bacteria such
as Pseudomonas aeruginosa (NCIM 2200), Staphylococcus aureus
(NCIM 5022), Streptococcus pyogenes (NCIM 2608), Clostridium
perfringens (NCIM 2677), Escherichia coli (NCIM 2065), Klebsiella
pneumonia (NCIM 5082), Klebsiella aerogens (NCIM 2239)
and fungal such as Candida albicans (NCIM 3471), Aspergillus
niger (NCIM 1196) were used as test organisms. The cultures
of organisms were procured from NCL (National Chemical
laboratory) Pune, India and tested. The petri plates were prepared
by pouring melted nutrient agar inoculated with 16 to 18 hr old
culture test organisms in a sterile petri dish. Cups were bored in
agar by means of sterile cork borer and were filled with either
extract to be tested or standard or control and incubated at 37
0C for 18-20 hours. Mixture of dimethyl sulfoxide and water were
used as control. Chloramphenicol was served as standard when
efficacy was tested against bacteria while fluconazole was served
as standard for fungi. Diameter of each zone of inhibition was
measured and compared with standard
In-vivo pharmacological activities
Methanol extract of Salvadora persica twig was formulated
into 1.5 % Carbopol 971 P NF gel by using extract (1%), ethanol,
propylene glycol, triethanolamine and distilled water. Prepared
gel was evaluated for skin irritation and wound healing activities.
Animals
Albino Wistar rats of either sex weighing 180-200g were
used for the study. The animals were procured from Haffkine
Biopharmaceuticals, Mumbai, India. All animals were housed in
polypropylene cages under standard experimental conditions
with 26+2 0C ambient temperature and 12 h light-dark cycle. The
animals were fed standard pellet diet and were provided water
ad libitum. All experimental protocols were approved by the
Institutional Animal Ethic Committee (CUSCP/IAEC/10 /2013) of
C. U. Shah College of Pharmacy, Santacruz (w), India.
Skin irritation studies
Skin irritation study was conducted on albino rats as per
OECD guide lines No. 404 (OECD, 2004) in order to evaluate
safety of prepared topical gel [18]. The back of the albino rats was
shaved to remove the fur carefully, 24 hours before application of
the sample. Prepared topical Carbopol gel containing methanol
extract of Salvadora persica twig was applied on the skin patches
of albino rats and the site of application in terms of erythema and
edema was examined at 24, 48 and 72 hours for changes in any
dermal reactions. The irritation index was calculated to assess
the irritation potential of the prepared Carbopol gel according to
Draize Test [19].
In vivo evaluation of wound healing
Incision and excision wound models were used to evaluate
the wound-healing activity of prepared topical Carbopol gel
containing methanol extract of Salvadora persica twig.
Grouping of animals
For excision and incision wound study, male Wister rats (160-
180g) were selected and were divided into four groups of six
animals each. Rats were anesthetized with sodium pentobarbitone
injection (45mg/kg, i.p.) and depilated at the predetermined site
before wounding. Animals were divided into four groups of six
animals each.
Treatment (Group I): Received with topical application of
Carbopol gel containing methanol extract of Salvadora persica
twig
Positive control (Group II): Received topical application of
standard drug ointment i.e. Betadine
Negative (vehicle) control (Group III): Received with topical
application of plain Carbopol gel
Negative control (group IV): Animals were left without any
treatment
For both excision and incision wound models, the treatment
groups were classified and treated in the same manner.
Excision wound model
An excision wound was inflicted by cutting away approximately
500mm2 full thickness of the predetermined area on the
anterior-dorsal side of each rat. Each rat was kept in a separate
polypropylene cage and was provided with food and water ad
libitum. All the test formulations were applied starting from day
0 till complete epithelialization. Wound-healing property was
evaluated by % wound contraction percentage and time of wound
closure. The wound area was measured immediately by placing
a transparent paper over the wound and tracing it out, area of
this impression was calculated using the graph sheet. The same
procedure is employed every fourth day and wound contraction
was expressed as percentage of contraction. The period of
epithelialization was calculated as the number of days required
for falling off of the dead tissue remnants without any residual raw
wound [20].
Incision wound model
A paravertebral long incision of 6 cm length were made
through the skin and cutaneous muscle.
After the incision was made, the two ends of the wound were
closed with interrupted sutures with stitches 1cm apart using
sterile surgical thread and a curved needle. Carbopol gel containing
methanol extract of Salvadora persica twig, plain Carbopol gel and
Betadine were applied for 9 days. The sutures were then removed
on the 8th post - wounding day and the breaking strength of 10-day
old wound was measured by tensiometer [21].
Statistical analysis
Results were expressed as means ± SEM (Standard Error of
The Mean). Comparisons between groups were performed using
one way ANOVA followed by Turkey’s pair-wise comparison test
on Graph Pad Instat 3 statistical software.
Results and Discussions
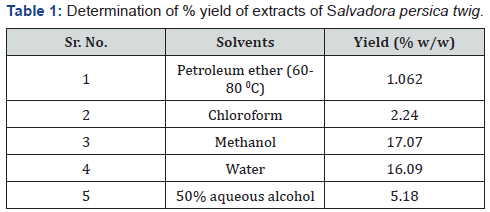
Extraction
The extraction process yielded 1.062 % w/w of petroleum
ether extract, 2.24 % w/w of chloroform extract, 17.07 % w/w
of the methanol extract, 16.09 w/w % of the of water extract and
5.18 % w/w of 50% aqueous alcoholic extract (Table 1 & 2).
in vitro antioxidant assay methods
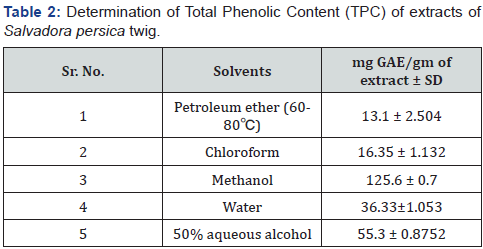
Determination of Total Phenolic Content (TPC): The
estimation of total phenolic content of the different extracts
revealed a high phenol content in the methanol extract i.e.
125.6±0.7mg/g gallic acid equivalent (GAE) followed by 50%
aqueous alcohol extract (55.3 ± 0.8752mg/g GAE), water extract
(36.33±1.053mg/g GAE), chloroform extract (16.35 ± 1.132mg/g
GAE ) and petroleum ether extract (13.1 ± 2.504mg/g GAE) by
reference to standard curve (y=0.011x+0.011and r2=0.998)
(Table 1).
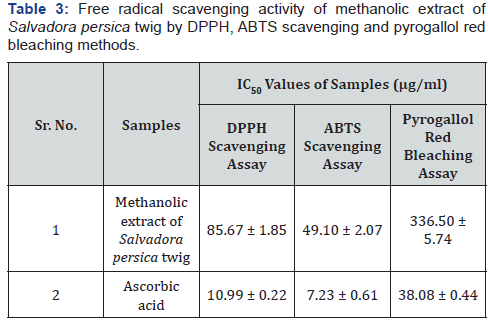
DPPH radical Scavenging activity: Anti-oxidant activity
of Miswak twig was studied by inhibition the stable free radical
DPPH. The amount of extract/standard needed for 50% inhibition
(IC50). Methanol extract of Salvadora persica twig showed DPPH
scavenging activity at higher IC50 value of 63.88 μg/ml as compared
to standard ascorbic acid (10.99 μg/ml).
Assessment of pyrogallol red bleaching by peroxynitrite:
The plant extract and standard exhibited inhibition of bleaching
by Pyrogallol Red method indicating peroxy nitrite scavenging
activity. Standard ascorbic acid was able to inhibit bleaching of
Pyrogallol Red at IC50 value of 38.08μg/ml. However methanol
extract of Salvadora persica twig showed less inhibitory activity
with IC50 value of 783.48μg/ml as compared to standard ascorbic
acid.
ABTS scavenging assay: Standard ascorbic acid was able
to scavenge ABTS radical at IC50 values of 7.23μg/ml. Methanol
extract of Salvadora persica twig exhibited moderate free radical
scavenging activity by ABTS method with IC50 values of 108.24μg/
ml.
In the present study antioxidant activity levels were found
to be relatively high in the methanolic extract of Miswak twig by
DPPH, ABTS and Pyrogallol bleaching method; hence oxidative
stress may be reduced which is associated with impaired or
delayed wound healing process (Table 3).
Antimicrobial activity
The methanol extract from Salvadora persica twig has shown
inhibition effects on the growth of all the organisms tested.
Amongst the test organisms used, Clostridium perfringens,
Candida albicans, Pseudomonas aeroginosa, Staphylococcus aureus
were found to be most sensitive to methanol extract of a Salvadora
persica twig followed by Klebsiella pneumoniae, Aspergillus niger,
Streptococcus pyogenes, Escherichia coli, Klebsiella aerogens.
Microbial infection of wounds delays healing and causes a more
pronounced acute inflammatory reaction which can lead to
further tissue injury and damage. The antimicrobial activity of
the extract on wound pathogens partly contribute to the wound
healing effect by eliminating infection thus allowing the natural
tissue repair processes to start. Hence the results of this study
confirm that the herbs possess anti-bacterial activity and this will
help keep the wound area sterile, thus promoting wound healing.
This fact supports a faster wound healing in the treated groups
compared with the control group (Table 4).

In vivo pharmacological activities
Skin irritation study: Carbopol gels containing methanol
extract of Salvadora persica twig showed no erythema or oedema
on intact rat skin. The primary skin irritation index of the gels
was calculated as 0.00. The results indicated that all Carbopol
gels did not cause any skin reaction after examining at 24, 48 and
72 hrs. Since the primary skin irritation index of the creams was
calculated as 0.00, it can be classified as non-irritant and were
found to be safe for topical application.
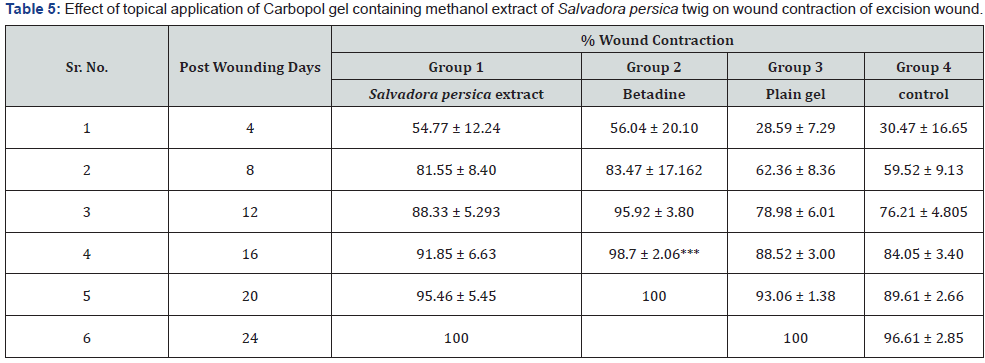
Excision Wound Study: Table 5 records the reduction of
wound area of the different groups over the period of 24 days.
It was seen that the faster healing of wound took place in case
of animals, which received Carbopol gel containing Miswak
extract of Salavadora persica twig and standard. The least rate
of wound healing was seen in control group (no treatment) and
vehicle control group which received plain Carbopol gel (without
extract). A very rapid closure of the wound in the both Carbopol
gel containing Miswak extract of Salavadora persica twig and
standard treated groups observed between 4 and 8 days of post
surgery. After day 8 of post surgery, wound closure was gradual till
the total closure of the wound. Total wound closure was observed
by the 22 day of post surgery in Carbopol gel containing methanol
extract of Salavadora persica twig and by 25 day in control group.
On 16th day, wound contraction of standard group was found to be
significant (p < 0.001) in comparison to control group. The period
of epithelization of standard group (16 days) was also found to
be significantly (p<0.001) low as compared to control group (25
days).
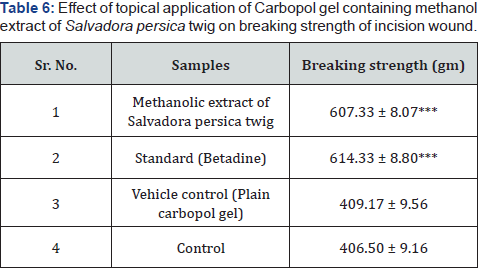
Incision wound model: Table 6 compares the tensile
strength of the healing skin treated with different gels measured
on 10th days. The results of the incision wound healing studies are
presented as mean weight in gram ± SD required to break open
the resutured wound. The animals treated with methanolic extract
and standard showed significant (p<0.001) increase in breaking
strength (607.33±8.07gm and 614.33±8.80 respectively) as
compared to the control group animals (406.50±9.16gm). This
observation confirms that the methanol extract of Salavadora
persica twig possesses excellent wound healing property so far as
tensile strength of wound healing tissue is concerned.
Conclusion
Results obtained in the present study have shown the
antioxidant and antimicrobial activity of methanol extract of
Miswak twig. Thus, the external application of methanol extract
of Salvadora persica twig on the wound prevented the microbes to
invade through the wound, resulting protection of wound against
the infections of the various microorganisms. At the same time,
external application of the extract entrapped the free radicals
liberated from the wound surrounding cells, which are having
inherent machinery to protect the cells from the microbes. The
faster rate of wound closer in excision wound model indicates
the better efficacy of medication the increase in tensile strength
of wounded skin indicates the promotion of collagen fibers. The
increased tensile strength reveals that the disrupted surfaces are
firmly knit by collagen. The synergistic effect of both antimicrobial
and antioxidant activity, increased wound contraction and
breaking strength accelerated the wound-healing process. Hence,
present study confirms the promising wound healing activity of
Salvadora persica twig.




Comments
Post a Comment