Lipid Peroxidation, Enzymatic and Non-Enzymatic Alterations of DCM-F of Rhizophora mucronata in Diabetic Rats- Juniper Publishers
Juniper Publishers- Journal of Complementary Medicine
Abstract
Oxidative stress is responsible for impairment of
β-cells caused by chronic glucose toxicity. The present study
demonstrates dichloromethane fraction (DCM-F) of Rhizophora mucronata
mediated fortification against diabetes mellitus induced alterations in
antioxidant defense system in the animal model. Single intraperitoneal
injection of streptozotocin and nicotinamide was to induce diabetes in
rats. 50mg/kg of DCM-F was orally treated to diabetic rats for 45 days.
At the end of the experiment, blood glucose, lipid hydro-peroxide (LH),
plasma enzymatic and non-enzymatic antioxidants were determined.
Treatment of DCM-F to the experimental rats notably (p<0.01) restored
blood-glucose, body weight, lipid profile and carbohydrate metabolic
enzyme activities. Besides, the intensity of LH increased and CAT,
glutathione peroxidase (GPx), GSH, and SOD were considerably decreased
in diabetic rats. These unfavourable alterations were inverted to normal
in DCM-F treated rats. Moreover, notable results on ceruloplasmin,
ascorbic acid, and tocopherol were observed in DCM-F treatment as
differenced with diabetic and control. In conclusion, DCM-F of R.
mucronata act as antioxidant linked with anti-hyperglycemic and act as a
ligand for selected antioxidant receptors.
Keywords:Antioxidant; DCM-F; Glutathione peroxidase; Oxidative stress, Rhizophora mucronateAbbrevations: CAT: Catalase; DCM-F: Dichloromethane Fraction; GPx: Glutathione Peroxidise; GSH: Glutathione; LH: Lipid Hydroperoxide; MDA: Malondialdehyde; PUFA: Polyunsaturated Fatty Acids; SOD: Superoxide Dismutase; TBARS: Thiobarbituric Acid Reactive Substances
Introduction
Free radicals produced during regular metabolism are
removed by way of an efficient scavenging system and the imbalance
effects in expanded oxidative strain. Lipid peroxide stages in diabetes
are extended in plasma, serum, kidney, lens and in erythrocyte membrane
[1]. Significant modifications in lipid metabolism and structural
modifications in cell membranes are related to the progress of metabolic
disorders [2]. The dysfunction among enzymatic and non-enzymatic
oxidation of lipids in vivo is not always absolute [3]. Oxidative
pressure has these days been proven accountable, as a minimum in the
component, for pancreatic β-mobile dysfunction due to glucose toxicity.
Under hyperglycemia, production of various decreasing sugars, which
includes glucose-6-phosphate and fructose, will increase through
glycolysis and polyol pathways [4]. During this method, reactive oxygen
species (ROS) are produced and cause tissue harm. So, STZ is broadly
hired to set off experimental diabetes in animals [5]. DCM-F of Rhizophora mucronata
is confirmed anti-hyperglycemic and anti-hyperlipidemic impact on
diabetic animals [6]. In the continuation of preceding research,
we have appraised the impact of DCM-F on lipid peroxidation and plasma
antioxidants in STZ-NAD induced diabetic animals.
Summary
Body weight, blood glucose, cholesterol, lipid
profile, plasma insulin and carbohydrate metabolic enzyme activities of
experimental rats were estimated and reported in the previous studies
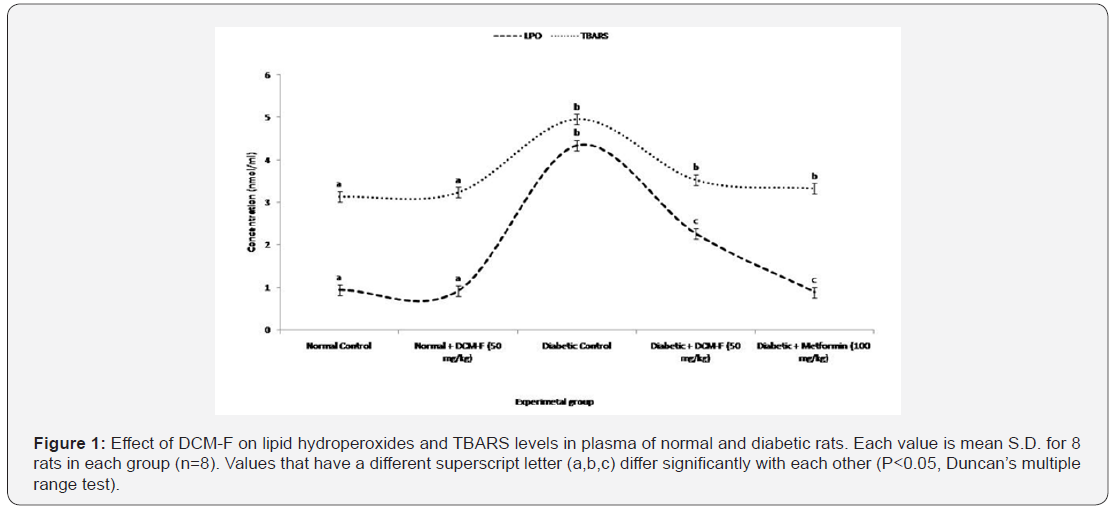
[6]. Figure 1 illustrated the level of LH and TBARS in the plasma of
experimental rats. TBARS concentrations were particularly raised in the
diabetic rats, distinguished with control and DCM-F treated rats. DCM-F
(50mg/kg) showed a convincing decrease in TBARS level. No consistent
changes were found among control and treated rats. Activities of
enzymatic antioxidants were clearly giveaway in diabetic rats compared
with control and treated rats. Lipid peroxidation is a process enhancing
the oxidation of polyunsaturated fatty acids, which causes metabolic
disorders, cancer, and cardiovascular diseases. The results of the
present study show STZ induced free radicals and increased oxidation of
PUFA in the plasma and tissues of diabetic rats. This confirms the
previous reports on the proficiency of STZ to assist production of free
radicals and causes
oxidative stress [7]. The increase of reactive oxygen species (ROS)
from the reaction of enzymes, metabolism of xenobiotics shows
the way to lipid peroxidation with consecutive cell disruption
and toxicity [8]. Free radical increases in liver and kidney
tissues may accommodate almost absorption of per-oxidizeable
fatty acids. DCM-F treatments reduce MDA, an indicator of
lipid peroxidation in the tissues of diabetic rats suggesting that
the DCM-F have massive anti-oxidative properties. Alkaloids of
mangroves have an important role as edible antioxidants for the
preclusion of oxidative damages in humans [9].
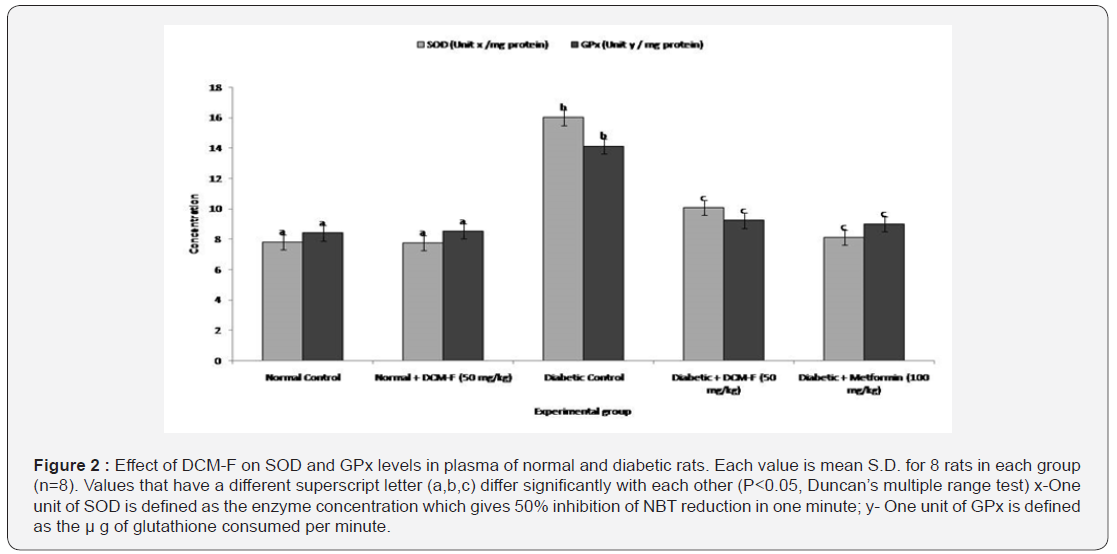
DCM-F treatment explained a rational (P<0.05) get higher
in the actions of superoxide dismutase, catalase (Table 1), and
glutathione peroxidise (Figure 2). Notable (P<0.05) increase
in the levels of glutathione, ascorbic acid, α-tocopherol and
ceruloplasmin in diabetic rats contrasted to control. DCM-F
treatment progress to a significant (P<0.05) increase in
the plasma concentrations of glutathione, ascorbic acid,
α-tocopherol, and ceruloplasmin if compared with diabetic
control rats (Table 1). No consistent variations were found among
control and treated rats. Catalase and superoxide dismutase
plays a major role in the reduction of highly reactive hydroxyl
radicals and dismutation of superoxide radicals respectively
[10-12]. Glutathione peroxidase detoxifies hydrogen-peroxide
into water through the oxidation of reduced glutathione
[13,14]. The present study results confirm the restored action of
enzymatic antioxidants through the treatment of DCM-F. In the continuation of lipid peroxidation and formation of hydrogen
peroxides are arisen. Inhibition of hydrogen peroxide means
that production of hydroxyl radicals is reduced which protects
the cells from xenobiotics [15]. In this view, antioxidant alkaloids
reduced the oxidative damages through inhibition of free radical
formation [16]. Depletion of glutathione may be accompanying
to the increased lipid peroxidation in the STZ induced diabetic
rats. Previous reports express decreased antioxidant enzyme
action enhanced peroxidative sta¬tus particularly liver and
kidney tissues of diabetic rats [17,18]. During peroxidation,
α-tocopherol reduces lipid hydroperoxides and protects cell
damage, while binding to the copper ion ceruloplasmin inhibits
lipid peroxidation [19]. Increased utilization as an antioxidant
defense system and reduced in the level of glutathione in diabetic
rats reduced ascorbic acid [20]. These are supports, DCM-F of
Rhizophora mucronata treatment restored antioxidant enzyme
action in diabetic rats through detoxification and scavenging
of free radicals. Rhizophora mucronata, the mangrove plant
isolated DCM-F was studied to control diabetes mellitus to
enhance the action of antioxidant enzymes. In conclusion, DCM-F
of Rhizophora mucronata act as antioxidant linked with antihyperglycemic
effect.
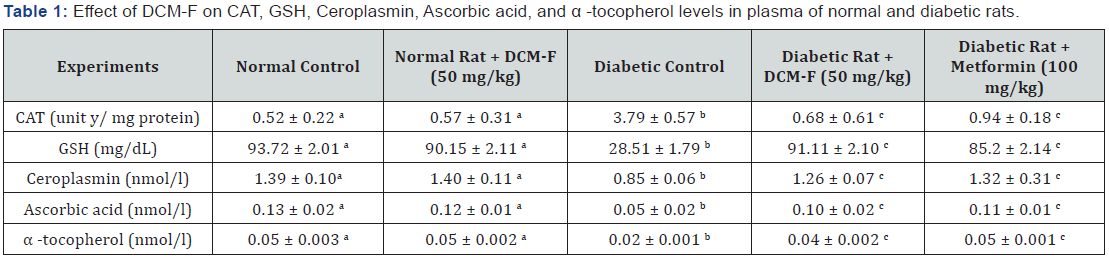
Values that have a different superscript letter (a,b,c) differ significantly with each other (P<0.05, Duncan’s multiple range test).
z-One unit of CAT is defined as the μ mole of hydrogen peroxide consumed per minute.
Acknowledgement
The authors are grateful to the University Grants Commission,
Govt. of India, New Delhi, India (UGC Ref. No.: 39-439/2010) for
financial support. The authors extend special thanks to Prof.
S. Sengottuvelu and Asst. Prof. V. Lalitha, Nandha College of
Pharmacy and Research Institute, TN, India for the support of
experimental section and data analysis.
For more Open access journals
please visit our site: Juniper Publishers
For more articles please click
on Journal of Complementary Medicine & Alternative Healthcare




Comments
Post a Comment