In-vitro Characteristics Comparison of Prepared Immediate Release Tablet of Linagliptin with Innovator Drug and Selected Market Samples of Bangladesh- Juniper Publishers
Juniper Publishers- Journal of complementary medicine
Abstract
The aim of the current investigation is to prepare
immediate release tablet of Linagliptin and then compare its
characteristics with innovator drug and marketed samples of Bangladesh.
Direct compression technique was applied to prepare immediate release
tablet of Linagliptin and physical characteristics as well as In-vitro
dissolution study was performed.Characteristics of prepared tablet,
innovator drug and marketed samples all were in official limit. Prepared
Linagliptin tablet had better immediate release profile than innovator
drug and marketed samples.
Keywords: Linagliptin;Immediate release;Innovator drug;Marketed samples
Introduction
Diabetes Mellitus is a chronic and incurable disorder
which affects a large number of populations and it is in increasing
trend[1]. If it is not managed properly than it will cause clinical,
social and economic complications. Diabetic patient has to live with
this disease and thus has to take medication during whole life which
eventually causes a large amount of cost in drug therapy. Linagliptin is
an effective agent which lowers blood glucose level. It is widely used
to treat type-2 diabetes and recommended to take once daily[2]. In the
meantime, oral route is considered as the most convenient route of
medication and getting action in a quickest possible time is one of the
main objectives of any medication. Basis on this, an attempt was made to
prepare immediate release tablet of Linagliptin and compare its
characteristics with innovator drug and marketed samples of the country
so that an idea can generate about the quality of prepared tablet.
Materials
Materials used in this experiment were of analytical
gradeand purchased from local market. Two marketed samples were
collected from retail pharmacies of Dhaka city, Bangladesh. Expiry date
was checked before collecting marketed samplesso as to ensure that those
samples are valid and can be used. Tablets of marketed samples were
purchased of same batch number.
Methods
Coding
Prepared tablets were coded as L-1, L-2 and L-3, innovator drug was coded as I and marketed samples were coded as M-1 and M-2.
Preparation of immediate release Linagliptin tablet
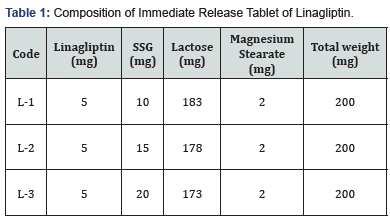
At first, drug and other excipients were weighed
according to the formula (Table 1) and then blended. After proper
blending and mixing direct compression technique was applied to prepare
the tablet[3-5].
Evaluation of physical properties of tablet
Existing official methods were followed in-order to find out
the characteristics of prepared tablet as well as the innovator drug
and marketed samples.
In-vitro dissolution study
In-vitro dissolution study was performed by taking tablets in
dissolution media which was 900ml of 0.N HCl (pH 1.2) solution.
Temperature was set 37±0.5℃and rpm was 50.5ml of solution
was taken in a definite time period for analysis and fresh solution
was transferred into the media. Withdrawn solution of samples
was analyzed at 299nm by using UV-visible spectrophotometer.
Results and Discussion
Physical properties of tablet
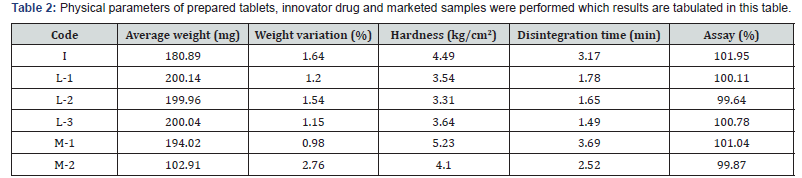
Physical parameters of prepared tablets, innovator drug and
marketed samples were performed which results are tabulated
inTable 2. It was found that, all were in the specification of official
limit.
In-vitro dissolution study
In-vitro dissolution result of prepared tablet, innovator drug
and marketed samples are presented in Table 3.
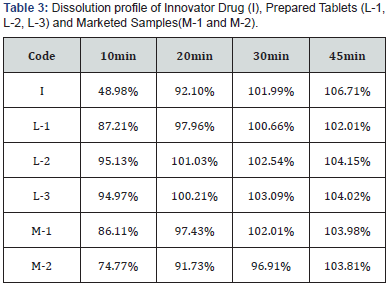
From Table-2, it is clear that, prepared Linagliptin tablets (L-
1, L-2 and L-3) have better release profile than the innovators
and marketed samples. 100% drug release was achieved first by
prepared tablets. L-1 achieved it in 30 min and both L-2 and L-3
achieved it in 20min. whereas, in case of marketed and innovator
sample, all took minimum 30min to release 100% of drug.
Conclusion
Immediate release tablet of Linagliptin was formulated
and compare with the innovator drug and marketed samples
successfully in the present study. It was also found that, quality of
the marketed samples were also up to the mark as their obtained
result were satisfactory. Commercialization of the investigated
immediate release tablets of Linagliptin will positively impact our
society as the number of diabetic patients is increasing day by day.




Comments
Post a Comment