Effect of Lycopene on Pharmacokinetic and Pharmacodynamics of Gliclazide in Diabetic Animal Models- juniper Publishers
Juniper Publishers- Journal of Complementary Medicine
Abstract
Background: Diabetes mellitus is one of the
metabolic disorders associated with high blood sugar levels. Utilization
of natural drugs among patients under diabetes mellitus pharmacotherapy
is across the board.
Objective: To examine the
pk-pd(pharmacodynamic-pharmacokinetic) interactions of lycopene and
gliclazide in animal models and to understand the safety &
effectiveness.
Methods: Single and multiple dose
interaction studies were carried out in normal rats, diabetes induced
rats and rabbits to evaluate the effect of lycopene on the gliclazide
activity. Blood samples from the study animals were used for the
estimation insulin and glucose levels by using radioimmunoassay method
and chemistry analyzer (automated) respectively. Homeostasis model
assessment used for determination of β-cell function. Additionally,
sophisticated HP-LC technique used for analysis of diabetic rabbits
serum samples for gliclazide.
Results: Gliclazide produces significant
reduction in blood glucose levels in diabetic animals. However, tests
examined from gliclazide in blend with lycopene indicated more prominent
diminishment in blood glucose concentration in animals with diabetes.
Conclusion: The study concludes that the
lycopene along with gliclazide shows the significant pharmacodynamics
interaction but doesn’t establish pharmacokinetics interaction up on
single and multiple-dose treatment in animals.
Keywords: Lycopene; Gliclazide; Diabetes mellitus; Pharmacokinetics; PharmacodynamicsAbbreviations: AUC: Area Under the Concentration Time Curve; AUMC: Area Under First Moment Curve; CL: Clearance; Cmax: Peak Serum Concentration; Kel: Elimination Rate Constant; MDT: Multiple-Dose Treatment; MRT: Mean Residence Time; SD: Standard Deviation; SDT: Single-Dose Treatment; Tmax: Peak Time; T1/2: Terminal Half-Life
Introduction
Multiple drug therapy is the simultaneous utilization
of different drugs. It can be related to the solution and additionally
utilization of excessive pharmaceuticals at measurements or frequencies
higher than remedially basic. These restorative combinations might be
lethal [1] or favorable [2] at the given therapeutic dose. Diabetes
Mellitus (DM) is a metabolic disorder signifies with blood sugar level
is abnormally high due to insulin insufficiency and function or both
[3]. Diabetic patient’s shows decreased antioxidant levels and increased
oxidative stress [4]. Gliclazide (second era sulfonylureas) is the
favored decision of medication which is accounted for to have to have
antioxidant properties [5] diminished inclination to prompt serious
hypoglycaemia and cell reinforcement properties [6]. The mechanism of
action includes K+ adenosine triphosphatase channel inhibition in
pancreas [7,8] and
gliclazide predominantly metabolized by CYP2C9 and moderately by CYP3A4
[9]. Indeed, phyto chemical extracts from herbs either alone or as
combination have been guaranteed to avert DM complications [10]. Of
these plants, mulberry (Morus alba L.) leaf, fenugreek (Trigonella foenumgraecum) seed [11], and American ginseng (Panaxquinquefolius) root [12] are much of the time proclaimed as worthy. Lycopene is a carotenoid, richest source in tomato fruits (Solanumlycopersicum)
[13]. It is also found in watermelon, papaya, pink grapefruit, and pink
guava [14]. A wide-ranging literature collection from all scientific
references revealed that lycopene has antioxidant [15], antidiabetic
activity and also a CYP3A4 enzyme inhibitor [16]. Reference gives
evidence for lycopene, most likely to prevent hepatocellular carcinoma
development [17], improvement in sperm quantity [18], motivates cell
proliferation to bone growth [19]. Nevertheless, there is
limited information about lycopene activity on blood glucose
levels and interaction with anti-diabetic drug gliclazide in animal
models. Therefore, this study innovates the hypoglycemic activity
of lycopene on gliclazide in animal models.
Methods
Drugs
Gift samples of gliclazide and lycopene acquired from DRL,
Hyderabad, India, and Parry Phytoremedies private limited, Pune
respectively. Alloxan (monohydrate) be procured from Loba
Chemie, Mumbai, India. Analytical grade materials and reagents
used for present study.
Lycopene solution
lycopene powder 20mg weighed and dissolved in distilled
water 10ml and make to 2 mg/ml solution. A dose of 4mg/kg of
body weight was administered by using clean and dry oral feeding
needle for 21 days [20].
Gliclazide solution
Gliclazide in a small amount of 0.1 N sodium hydroxideused
to prepare gliclazide solution and water used for final volume
makeup [21].
Preparation of alloxan solution
A dose of 110 mg/Kg alloxan monohydrate in sterile saline
prepared and injected by s.c. route instantly within few minutes to
evade degradation [20].
Animals
Albino rats (8-9 weeks aged) & albino rabbits (3 months
old) selected gender male aged having weight between 170 and
250g and between 1 and 1.5kg respectively were obtained from
M/s Mahavir Enterprises, Hyderabad. They were maintained
under controlled room temperature (24±20C; relative humidity
60-70%) in a 12h light - dark cycle. The animals were provided a
standard laboratory diet and water ad libitum. Before performing
the experiment, the animals were acclimatized. The experimental
protocol was approved by the Institutional Animals Ethics
Committee (IAEC). Reference # GBN/GQ/2014.
Experimental study design
Five groups of male albino rats/rabbits were made and each
consisting of six animals. Based on the gliclazide doses of 2 and
4mg/kg per body from dose-effect association learning in normal
rats and rabbits, the weight considered for oral administration.
Study designed as follows:
Group I: Normal control
Group II: Diabetic control
Group III: Gliclazide (2mg/kg for rats/; 4mg/kg for rabbits)
body weight, po.
Group IV: 4mg/kg, po of Lycopene on body weight.
Group V: Lycopene (4mg/kg) + Gliclazide (2mg/kg for
rats/4mg/kg for rabbits) body weight, po.
Diabetic rats - pharmacodynamic interactions
Male albino rats weighing (170-250g) were fasted for
overnight prior to administration of freshly prepared alloxan
monohydrate solution and injected within 5min of preparation to
avoid solution degradation at a dose of 110mg/kg. Immediately,
5% glucose solution was orally administered for 72h to prevent
any chances of hypoglycemia shock. Animals had provided with
continuous with water and feed. Animals were confirmed for
development of hyperglycaemia by analysis of fasting serum
glucose levels after 72h of alloxan monohydrate solution injection
where animals were fasted for a second time of 14h and blood
samples collected from retro orbital plexus.
The rats having a fasting blood glucose level of 200mg/dl
or above at 72h were included in the study as diabetic subjects.
Gliclazide (2mg/kg, po.) was administered after 30min of
lycopene administration (4mg/kg, po.). Blood glucose levels were
estimated on initial, 1st day, 3rd day, 7th day, 14th day, and 21st day of
the treatment.
Normal rats - pharmacodynamic interactions
Six rats were chosen for the investigation. Gliclazide (2mg/
kg of animal body weight) was given orally to Group III rats and
withdrawn blood at programmed time points. A similar procedure
was followed with either lycopene 4mg/kg, po. (Group IV) alone
or combination of lycopene and gliclazide (Group V) as per the
designed doses. Consequently, the treatment was sustained for 20
days with standard feeding. The animals were dosed with gliclazide
(2mg/kg) after 30minutes of each lycopene treatment. Samples
withdrawn at programmed time points for each treatment [9].
Diabetic rabbits - pharmacodynamic & pharmacokinetic interactions
Each group has six rabbits. Alloxan (100mg/kg) in normal
saline used to induce diabetes on single intravenous iv injection
[22]. Gliclazide (4mg/kg) was given orally to Group III rabbits
and their samples withdrawn at planned time points. A similar
procedure was followed with either lycopene only (Group IV) or
combination of lycopene and gliclazide (Group V) at the specified
doses.
After this single-dose interaction study, the same animals
received daily treatments with lycopene for the next 20 days with
regular feeding. Before 30minutes of gliclazide treatment the
animals were dosed with lycopene for each treatment. Samples
withdrawn at scheduled time intervals for each treatment of drugs
gliclazide, lycopene, or combination [23].
Collection of serum samples
Under light ether anesthesia, marginal ear vein and retro
orbital plexus punctured and the blood withdrawnfor14h fasted
rats and rabbits respectively on different occasions i.e., day
0, 1st day, 3rd day, 7th day, 14th day, and 21st day. On day 0 (SDT)
and day 21st (MDT) blood samples collected at different time intervals as 0h, 1h, 2h, 4h, 8h, 10h and 12h for pharmacokinetic
study experiment and to estimate insulin levels and glucose
concentration by using radioimmunoassay and chemistry analyzer
(automated), respectively. Homeostasis model assessment was
used to determine β-cell function. Additionally, high-performance
LC (Liquid chromatography) technique was used and determines
the serum gliclazide concentration in rabbits.
Determination of β-cell function
β-cell function was assessed by the homeostatic model
assessment protocol and calculated [21,24,25].
β-cell function = (FSI x 20) / (FSG - 3.5) ×100
Where FSI=fasting serum insulin (μIU/ml) and
FSG= fasting serum glucose(mg/dL).
Pharmacokinetics: Gliclazide pharmacokinetics estimated
by Kinetica 5.0 software, Alfa soft Limited.
Statistical analysis: The data were analysed using one-way
analysis of variance (ANOVA), followed by Dunnett’s test (Sigma
Plot version 11) and p<0.05 was considered as statistically
significant. The data were expressed as mean ± Standard Deviation
(SD).
Results
Pharmacodynamic interaction between lycopene and gliclazide
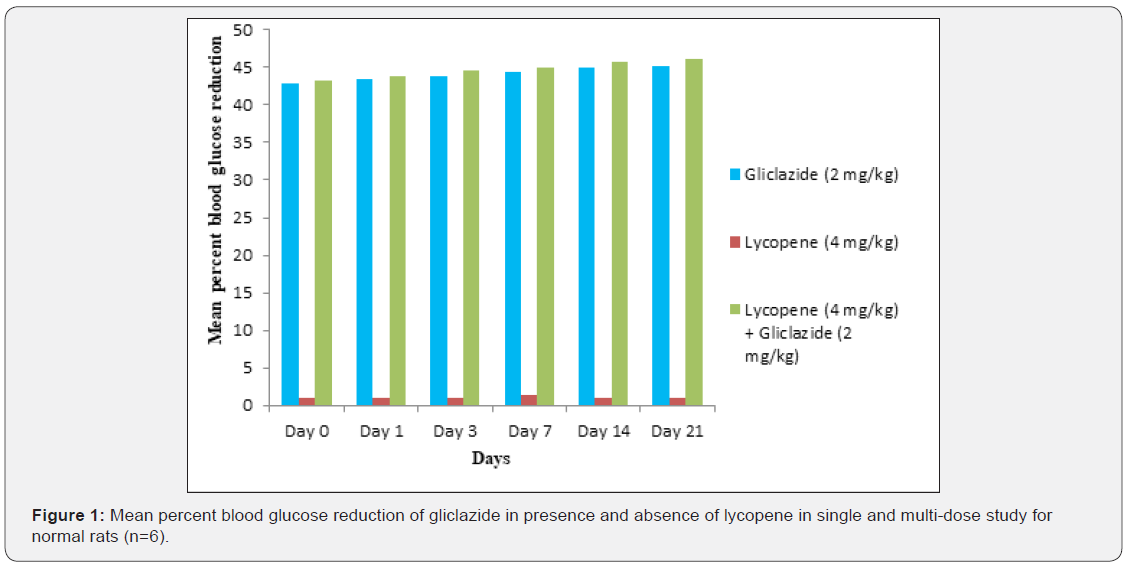
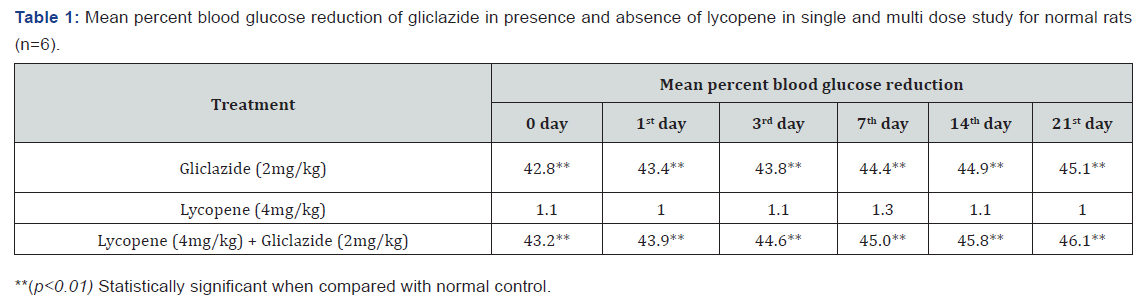
Gliclazide results: Gliclazide created the hypoglycemic
effect in normal rats. The observations from Table 1 & Figure 1 of
lowering of blood glucose levels by improvement in mean percent
blood glucose reduction from initial day 42.8% to 45.1% for the
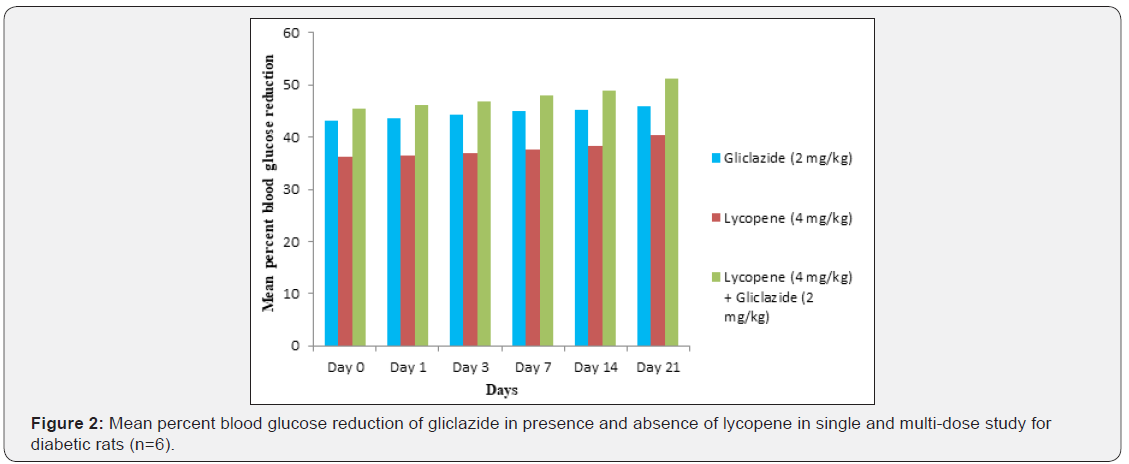
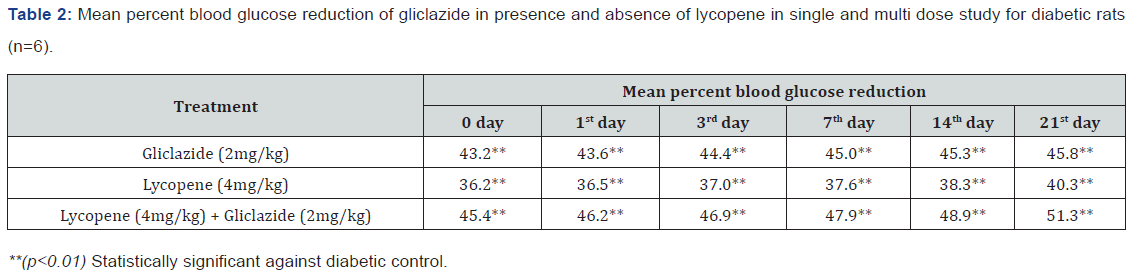
day 21 treatment. The results from Table 2 & Figure 2 in diabetic
rats the activity is more extreme as per record mean percentage
blood glucose reduction from initial day 43.2% to 45.8% on day
21 treatment. In diabetic rabbits the anti-hyperglycemias action
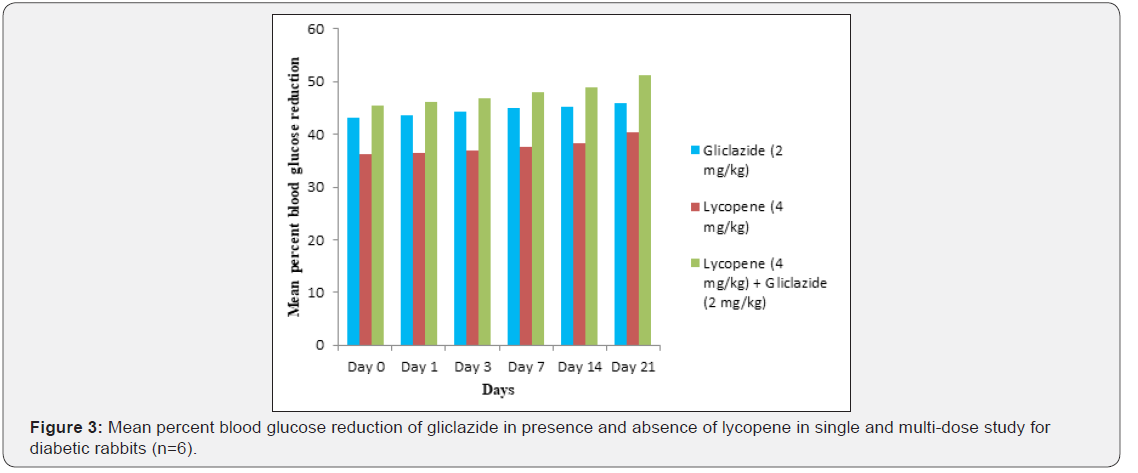
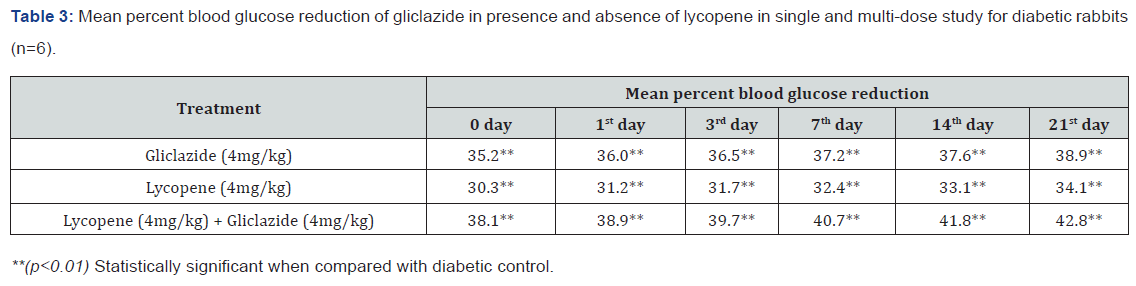
was shown in Table 3 and Figure 3 with maximum percent blood
glucose reduction of 35.2% to 38.9% from the initial day to 21st
day respectively. The anti-hyperglycaemic activity evidenced by
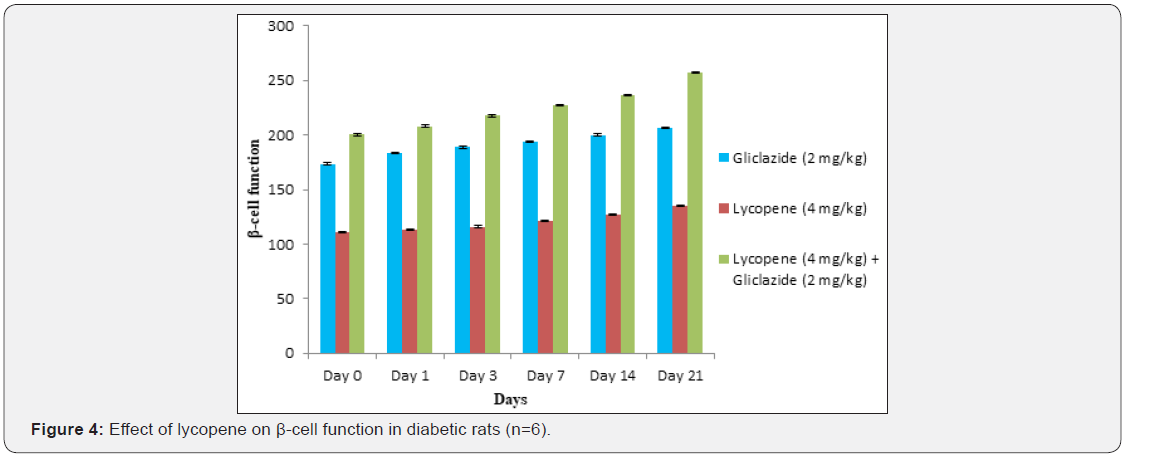
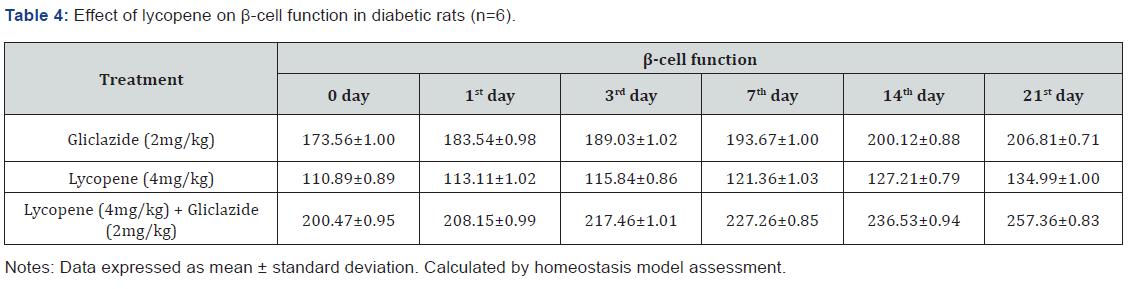
improvement in β-cell function and insulin levels as recorded
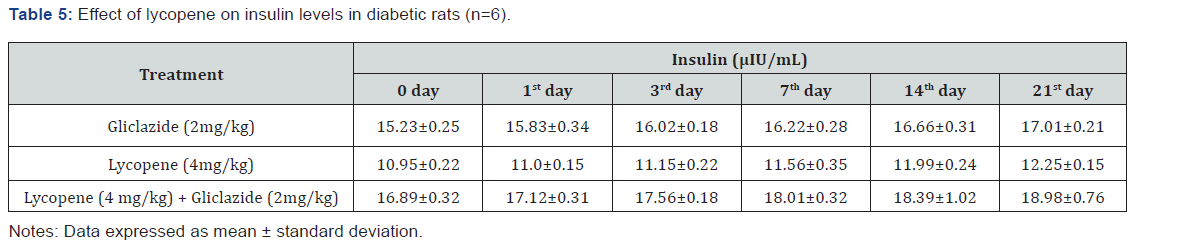
in (Table 4 & 5) & (Figure 4 & 5) for the initial dose in the 21st
day study are 173.56±1.00 to 206.81±0.71 and 15.23±0.25 to
17.01±0.21 respectively in diabetic rats.

Lycopene results
The blood glucose levels from Table 1 and Figure 1 in normal
rats doesn’t alter by lycopene treatment alone, but significant mean
percentage blood glucose reduction observed in diabetic rabbits
as 36.2% to 40.3% from day initially to 21st day treatment from
Table 2 & Figure 2.This is the similar fashion as with gliclaizde the
anti-hyperglycaemic activity probably with improvement in β-cell
function and insulin levels as recorded in (Table 4&5) & (Figure
4&5) for the initial dose to 21st day study are 110.89±0.89 to
134.99±1.00 and 10.95±0.22 to 12.25±0.15 respectively.
Combination treatment results
Single and multiple dosed lycopene along with gliclazide
proved to significant percent blood glucose improvement as 43.2%
of initial dose to 46.1% of 21st day treatment in normal animal
models (Table 1 & Figure 1). Further, numerous measurement
combination of lycopene with gliclazide created fundamentally
more prominent decreasing in blood glucose levels after treatment
in diabetic rats and rabbits when contrasted and diabetic control.
The values recorded from an initial dose to 21st day treatment of
mean percentage blood glucose reduction are 45.4% to 51.3%
and 38.1% to 42.8% of diabetic rats from Table 2 & Figure 1 and
rabbits Table 3 & Figure 3 respectively.
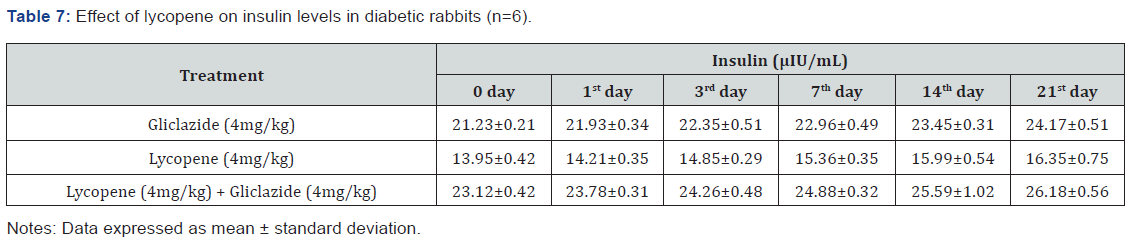
Lycopene exhibited supportive effect by escalating the
activity of gliclazide and also significant changes in β-cell function
200.47±0.95 to 257.36±0.83 and insulin levels16.89±0.32 to
18.98±0.76μIU/mL in diabetic rats (Table 4&5 and Figure 4&5).
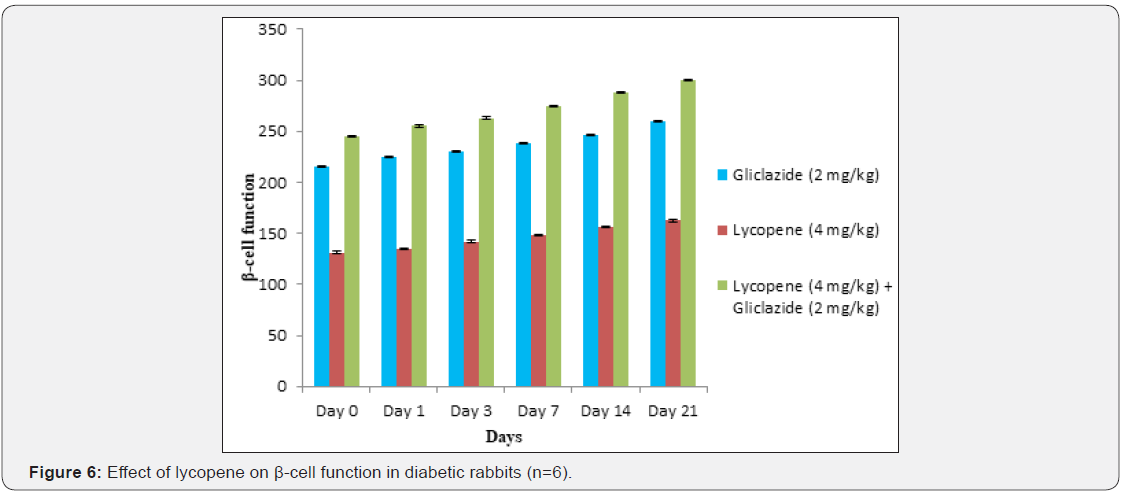
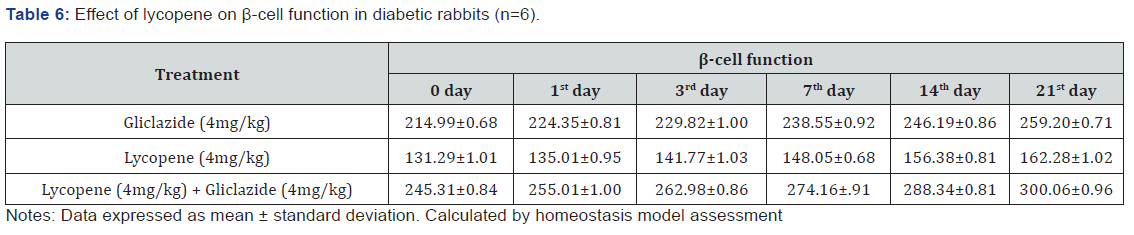
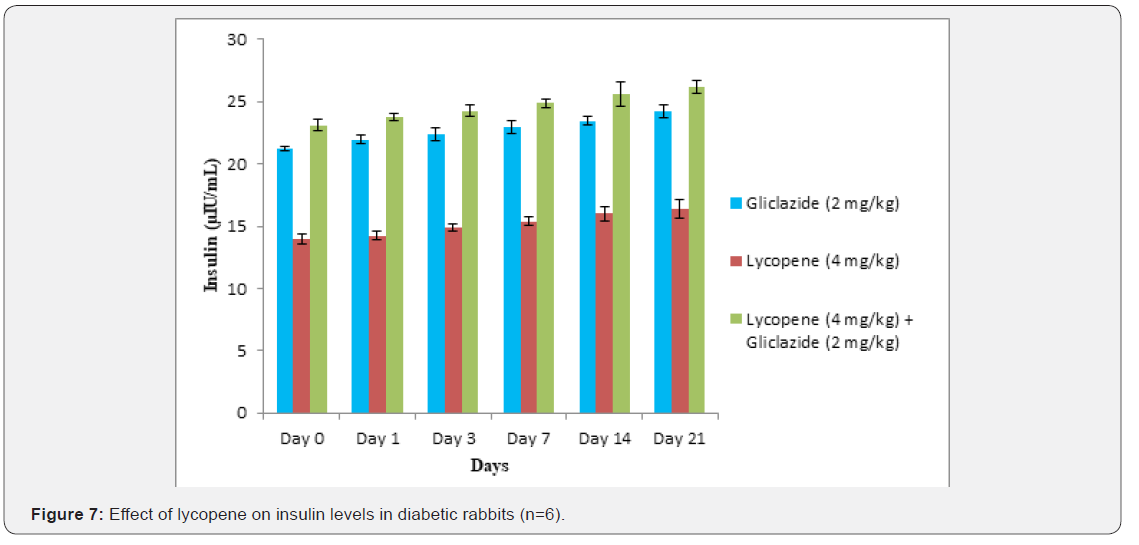
Whereas the results validated from diabetic rabbit study the
significant changes in β-cell function 200.47±0.95 to 257.36±0.83
and insulin levels 23.12±0.42 to 26.18±0.56μIU/mL from initial
dose of single dose study to 21st day dose of multiple dosed study
represented in Table 6 & 7 and (Figure 6&7).
Pharmacokinetic interaction between lycopene and gliclazide
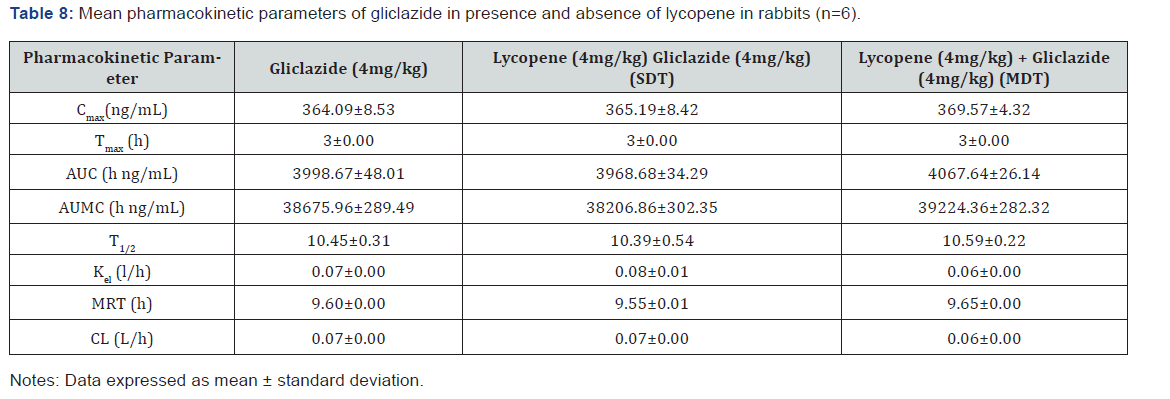
Pharmacokinetic parameters for gliclazide alone and along
with lycopene on single and multiple-dose administration were
recorded as given Table 8. There are no major fold changes with
the combination treatment in diabetic rabbits.
Discussion
Medication combinations believe about being an imperative
part of pharmacology research, and such communications are
normally assessed in animal models [26]. Even though animal
models can never swap the requirement for far reaching thinks
about in human subjects, they help in understanding the
systems of medication collaborations. The present investigation
is intended to assess the impact of lycopene on the action of
gliclazide in animal models. Animal model using normal rats was
used to identify the interaction where as that of diabetic rats was
used to validate the interaction. The experiment further validated
by using dissimilar species, rabbit model [23]. The hypoglycemic
action of gliclazide in rats is interceded by blocking K+channels
in β-cells of pancreas [27], in this way empowering insulin
emission and as well as improvement in β-cell function by the
way expanding tissue take-up of glucose [28]. Insulin levels were
estimated at time intervals, where greater improvement in mean
percent blood glucose levels were observed both in rats and in
rabbits under diabetes upon gliclazide treatment. Any medication
or herbal active component may change the pharmacokinetic
and pharmacodynamic movement of the substrate when it is a
potential inducer or inhibitor of that specific medication utilizing
proteins such as metabolizing enzymes. Lycopene has the
potential to cause herb-drug interaction when administered with
other drugs. This study revealed the influence of lycopene on the
pharmacodynamic activity of gliclazide alone and in combination
using single and multiple-dose treatments in rats and rabbits.
The end determinations were assessed as far as glucose level
(% mean glucose reduction), insulin level and β-cell work
utilizing homeostatic model assessment and pharmacokinetics
of gliclazide in rabbits. In the present investigation, single and
multiple-dose treatment of lycopene obtained about significant
change via improvement with percent blood glucose reduction in
diabetic rats and diabetic rabbits when contrasted and normal and
diabetic controls individually. Here lycopene shows a supportive
effect when combined with gliclazide. Liver has been appeared
to be an insulin subordinate tissue, and is apparently engaged with glucose and lipid homeostasis, which is normally extremely
influenced amid diabetes [29]. Insulin impacts the intracellular
usage of glucose in various ways. Glucokinase catalyzes the change
of glucose to glucose - 6-phosphate and assumes a focal part in the
preservation of glucose homeostasis. In the liver, this compound
is an imperative controller of glucose stockpiling and transfer
[30]. Lycopene may exert its hypoglycemia activity via increased
hepatic glucokinase activity and probably by stimulating insulin
release from pancreatic β-cells as evidenced by elevated serum
insulin level [15]. Lycopene also evidenced for potent inhibitory
effect on CYP3A4 enzyme [16]. One compartment open model
was utilized to assess the pharmacokinetic parameters. Lycopene
might not have any huge impact on the metabolism of gliclazide,
which is fundamentally used by CYP2C9 and moderately by
CYP3A4. This could most likely clarify the outcomes got in this
examination where the pharmacokinetics of gliclazide was not
changed by lycopene in both single-and different measurements
treatment considers.
Conclusion
The examination affirms that the interaction of lycopene with
gliclazide is pharmacodynamic in nature as the glucose levels in
animal models are significantly reformed and no correction in
pharmacokinetics of gliclazide is observed. Since the interaction
is grasped in two different species, it is likewise liable to happen
in people. Hence, this combination needs observing of glucose
levels occasionally when managed for their clinical advantages in
diabetic patients.




Comments
Post a Comment